Changing States of Matter
Explore how matter transforms between solids, liquids, and gases through energy changes, and understand that mass remains constant throughout these physical processes.
1 The Basics: Solids, Liquids, and Gases
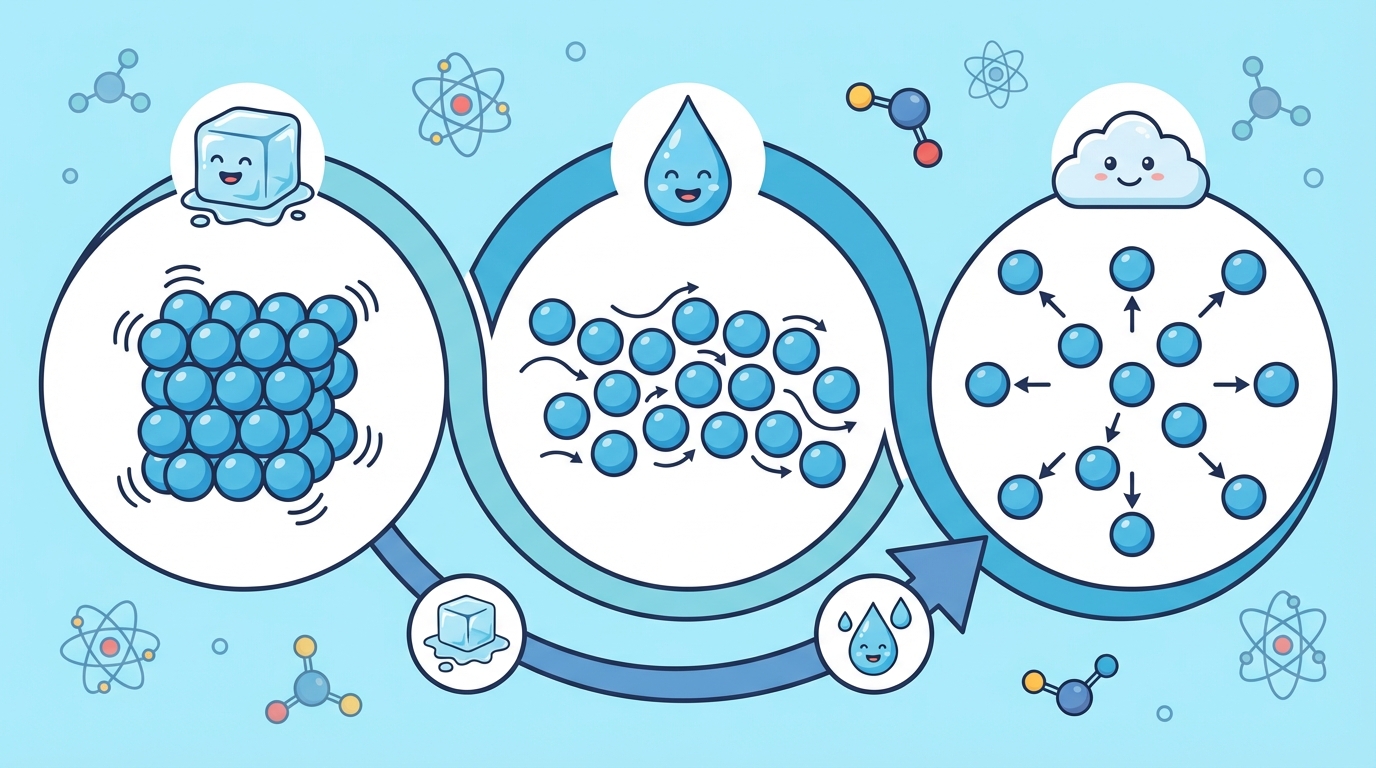
Everything around you—from your pencil to the water you drink—is made of matter. Matter is made up of tiny particles called atoms and molecules. How these particles move determines if something is a solid, liquid, or gas!
Solids have a fixed shape and volume. The particles are packed tightly together like a crowded elevator and only vibrate in place.
Liquids have a fixed volume but change shape to fit their container. The particles are close but can slide past each other.
Gases have no fixed shape or volume. They expand to fill any space! The particles move very fast and are far apart.
🔬 Particle Behavior Comparison
| State | Shape | Volume | Particle Movement |
|---|---|---|---|
| Solid | Fixed | Fixed | Vibrate in place |
| Liquid | Takes container shape | Fixed | Slide past each other |
| Gas | Fills container | Changes | Move freely & quickly |
Key Facts
2 It's All About Energy: Heat and Particles
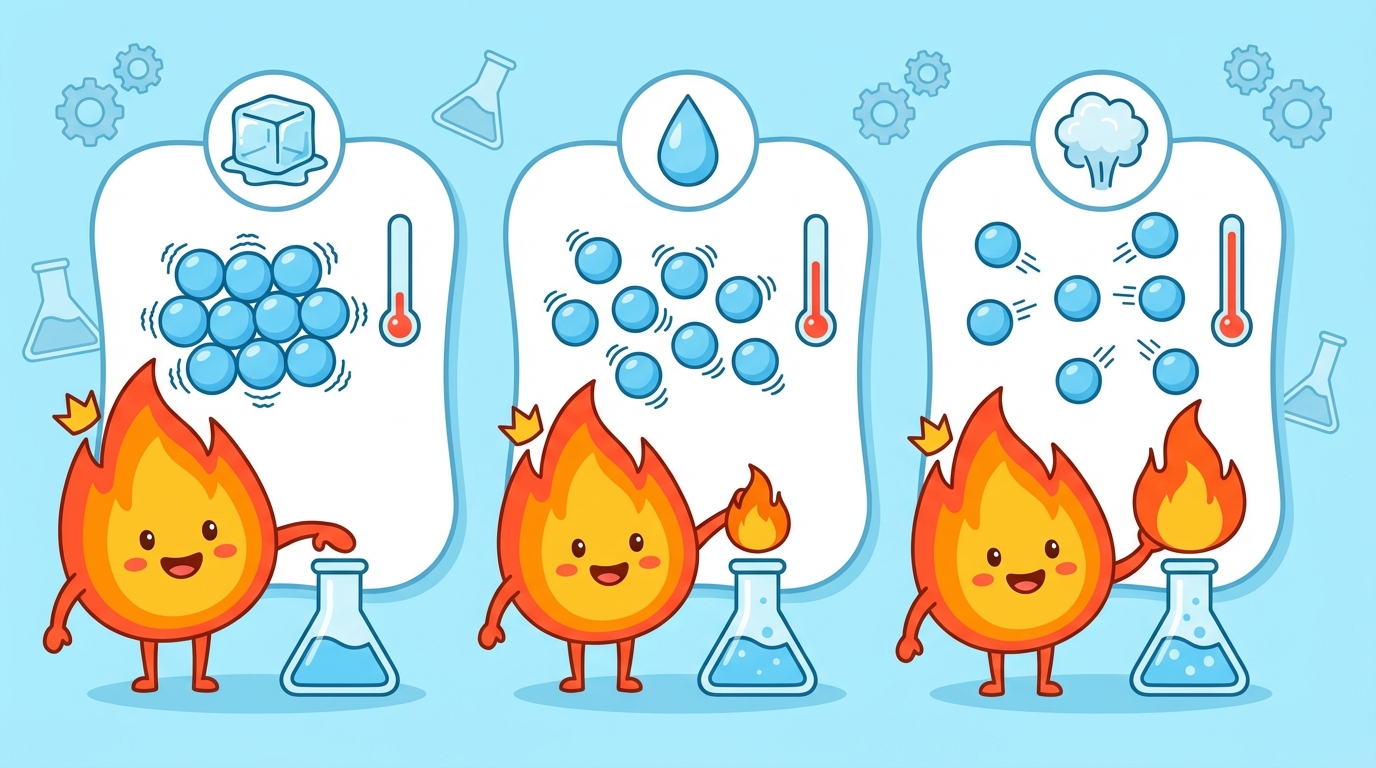
Matter is like a shapeshifter! 🧊 💧 💨 Whether something is a solid, liquid, or gas depends entirely on how much energy its particles have.
When you add heat, particles get excited and move faster and further apart.
- Melting: Solid ➔ Liquid.
Example: An ice cube turning into water. - Evaporation: Liquid ➔ Gas.
Example: Boiling water for pasta.
When you remove heat, particles lose energy, move slower, and clump together.
- Condensation: Gas ➔ Liquid.
Example: Dew on grass in the morning. - Freezing: Liquid ➔ Solid.
Example: Making popsicles in the freezer.
🧪 Weird Science: Sublimation
Did you know? Sometimes a solid can turn directly into a gas, skipping the liquid phase entirely! This is called Sublimation. Dry Ice (frozen carbon dioxide) does this to create spooky fog effects!
Key Facts
3 Melting and Freezing: Solids and Liquids
Have you ever wondered why your ice cream drips on a hot day or how puddles turn into ice skating rinks? It's all about energy! ⚡
🔥 Melting (Solid to Liquid)
Melting is the process where a solid turns into a liquid. This happens when a solid absorbs heat energy.
- The particles start vibrating faster.
- They break away from their fixed positions.
- Example: An ice cube turning into a puddle of water at 0°C (32°F).
❄️ Freezing (Liquid to Solid)
Freezing is the reverse! It is the process where a liquid turns into a solid. This happens when a liquid loses heat energy.
- The particles move slower.
- They lock into a fixed, rigid structure.
- Example: Making fruit juice popsicles in the freezer.
Comparison: What's the difference?
| Feature | Melting 🍦 | Freezing 🧊 |
|---|---|---|
| Direction | Solid ➡️ Liquid | Liquid ➡️ Solid |
| Energy | Heat is Added (Gained) | Heat is Removed (Lost) |
| Particle Speed | Speed Up | Slow Down |
Key Facts
4 Evaporation and Condensation: Liquids and Gases

Have you ever wondered why puddles disappear after the rain, or why a cold soda can gets wet on the outside? It's all about energy and moving particles! 🏃♂️💨
Evaporation happens when a liquid turns into a gas. This usually requires heat energy.
Imagine the particles in a cup of water. When they heat up, they start moving faster and faster. Eventually, the particles on the surface get so much energy that they break free and float away into the air as water vapor!
- ☀️ Example: Wet clothes drying in the sun.
- 🍝 Example: Boiling water for pasta (rapid evaporation).
Condensation is the opposite! It happens when a gas turns back into a liquid. This occurs when gas particles cool down.
When water vapor in the air touches a cold surface, the particles lose energy. They slow down, come closer together, and turn back into liquid water droplets.
- 🥤 Example: Water droplets on a cold drink.
- 🌫️ Example: Fog on the bathroom mirror after a shower.
🔄 The Great Exchange!
| Feature | Evaporation | Condensation |
|---|---|---|
| Change | Liquid to Gas | Gas to Liquid |
| Energy | Heat is added (Gets warmer) | Heat is lost (Gets cooler) |
| Particles | Speed up & spread out | Slow down & clump together |
Key Facts
5 Skipping Steps: Sublimation and Deposition
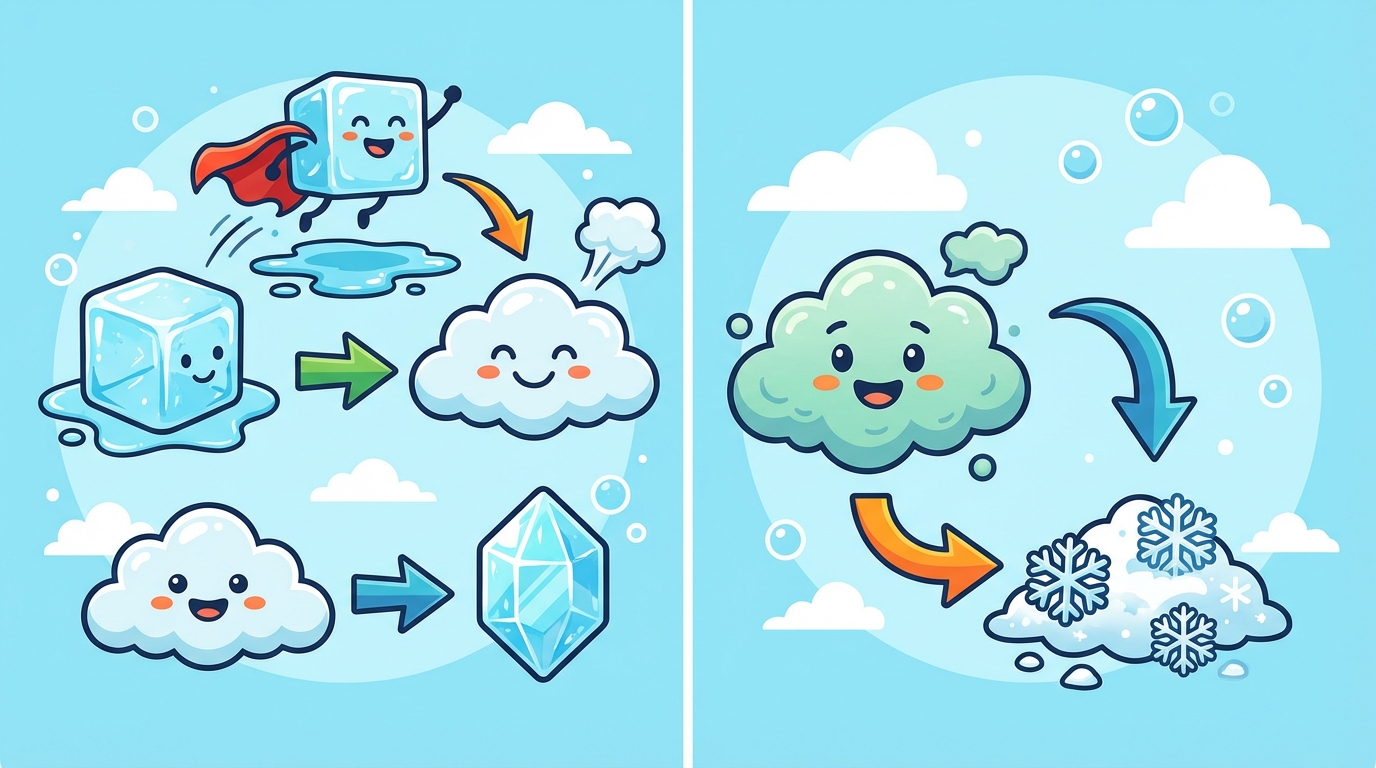
Usually, matter follows the rules: solids melt into liquids, and liquids boil into gases. But sometimes, matter is in a hurry and skips a step completely! 🏃💨
🔥 Sublimation (Solid ➔ Gas)
Sublimation occurs when a solid turns directly into a gas without ever becoming a liquid puddle. This requires a lot of energy (heat)!
Dry Ice is frozen carbon dioxide. At room temperature, it doesn't melt—it sublimates into a cool, spooky white fog. This is used in stage effects! 🎭
❄️ Deposition (Gas ➔ Solid)
Deposition is the exact opposite. It happens when a gas turns directly into a solid. This happens when heat is removed very quickly.
On a freezing morning, invisible water vapor in the air touches cold grass or windows and instantly turns into ice crystals (frost) without becoming water first. 🌿
Comparison: The Shortcuts
| Process | Change | Energy Needs |
|---|---|---|
| Sublimation ⬆️ | Solid ➔ Gas | Needs Heat (Absorbs Energy) |
| Deposition ⬇️ | Gas ➔ Solid | Loses Heat (Releases Energy) |
Key Facts
6 Conservation of Mass: Matter is Never Lost
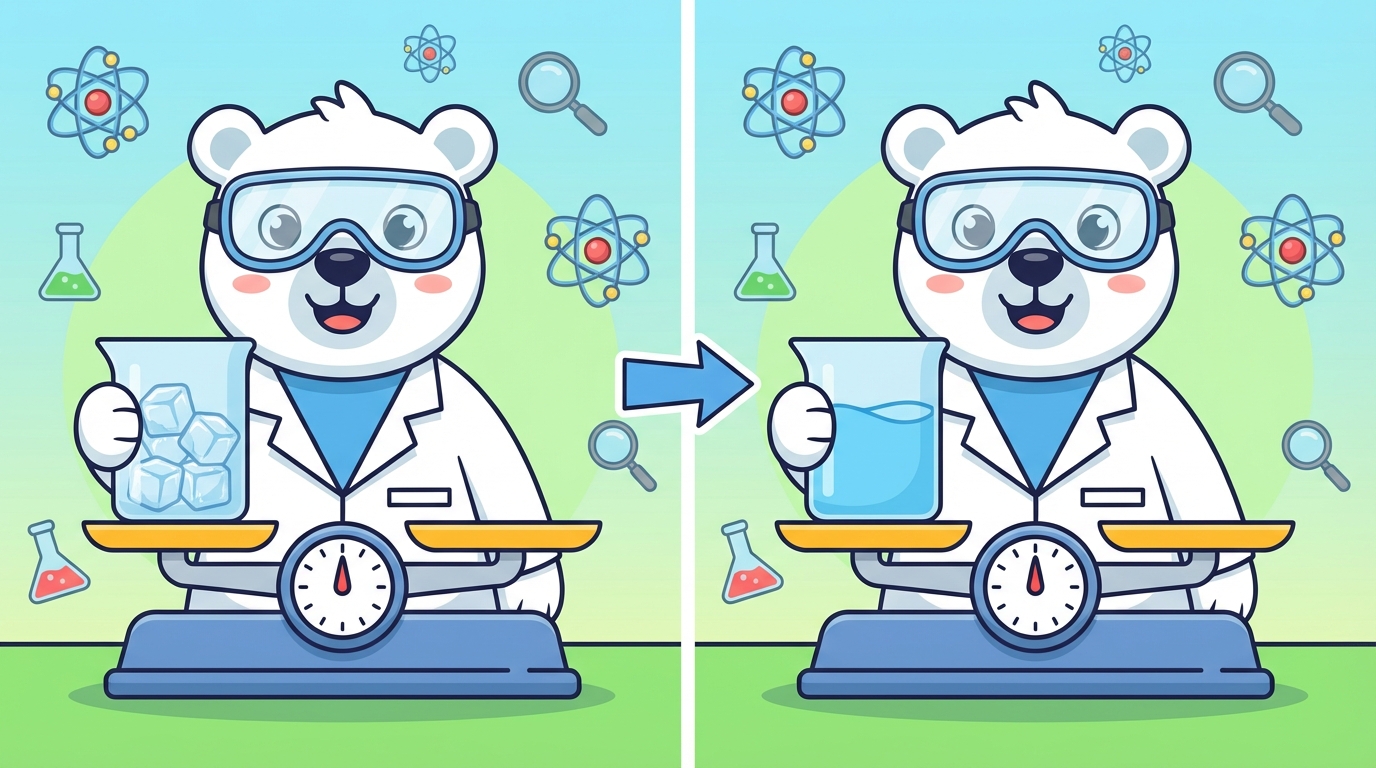
Have you ever wondered if a glass of ice weighs less after it melts into water? 🧊 ➡️ 💧 The answer is a big scientific NO!
⚖️ The Golden Rule: Law of Conservation of Mass
This is one of the most important rules in the universe. It states that matter cannot be created or destroyed; it only changes form.
Think of the particles (atoms and molecules) like students in a classroom. Whether they are sitting at their desks (Solid), walking around (Liquid), or running at recess (Gas), the number of students stays the same. None of them disappear!
🧱 The LEGO® Analogy
Imagine building a castle with 100 LEGO bricks. If you take the castle apart and put the bricks in a pile, do you have fewer bricks? No! You still have 100 bricks. The shape changed, but the mass (amount of stuff) remained the same.
🧪 The Sealed Jar Experiment
If you put an ice cube in a jar, seal the lid tight, and weigh it, let's say it's 200 grams. After the ice melts completely, if you weigh it again, it will still be exactly 200 grams. The water didn't escape!
Tracking the Mass
| State of Matter | What Happens? | Mass (Weight) |
|---|---|---|
| 🧊 Solid (Ice) | Particles vibrate in place | 100 grams |
| 💧 Liquid (Water) | Particles slide past each other | 100 grams |
| 💨 Gas (Steam) | Particles fly far apart* | 100 grams |
*Note: For gas, you must catch all the steam in a balloon or jar to prove the weight is the same!
Key Facts
7 Key Vocabulary
Master these important terms for your exam:
| Term | Definition |
|---|---|
|
Matter
Materia |
Anything that has mass and takes up space.
Cualquier cosa que tiene masa y ocupa espacio. |
|
Solid
Sólido |
A state of matter with a fixed shape and volume; particles vibrate in place.
Un estado de la materia con forma y volumen definidos; las partículas vibran en su lugar. |
|
Liquid
Líquido |
A state of matter with a fixed volume but no fixed shape; it takes the shape of its container.
Un estado de la materia con volumen definido pero sin forma fija; toma la forma de su recipiente. |
|
Gas
Gas |
A state of matter with no fixed shape or volume; particles move freely and quickly.
Un estado de la materia sin forma ni volumen definidos; las partículas se mueven libre y rápidamente. |
|
Particle
Partícula |
A very small piece of matter that makes up everything around us.
Una pieza muy pequeña de materia que compone todo lo que nos rodea. |
|
Kinetic Energy
Energía Cinética |
The energy an object has due to its motion.
La energía que tiene un objeto debido a su movimiento. |
|
Thermal Energy
Energía Térmica |
The total energy of the moving particles in an object (heat energy).
La energía total de las partículas en movimiento dentro de un objeto (energía calorífica). |
|
Temperature
Temperatura |
A measure of the average kinetic energy of the particles in a substance.
Una medida de la energía cinética promedio de las partículas en una sustancia. |
|
Melting
Fusión |
The change of state from a solid to a liquid.
El cambio de estado de sólido a líquido. |
|
Freezing
Solidificación |
The change of state from a liquid to a solid.
El cambio de estado de líquido a sólido. |
|
Evaporation
Evaporación |
The process where a liquid turns into a gas at the surface of the liquid.
El proceso donde un líquido se convierte en gas en la superficie del líquido. |
|
Boiling
Ebullición |
The process where a liquid turns into a gas throughout the liquid, creating bubbles.
El proceso donde un líquido se convierte en gas en todo el líquido, creando burbujas. |
|
Condensation
Condensación |
The change of state from a gas to a liquid.
El cambio de estado de gas a líquido. |
|
Sublimation
Sublimación |
The change of state directly from a solid to a gas without becoming a liquid first.
El cambio de estado directamente de sólido a gas sin pasar primero por líquido. |
|
Deposition
Deposición |
The change of state directly from a gas to a solid without becoming a liquid first.
El cambio de estado directamente de gas a sólido sin pasar primero por líquido. |
|
Melting Point
Punto de Fusión |
The specific temperature at which a solid becomes a liquid.
La temperatura específica a la cual un sólido se convierte en líquido. |
|
Boiling Point
Punto de Ebullición |
The specific temperature at which a liquid becomes a gas.
La temperatura específica a la cual un líquido se convierte en gas. |
Time to Practice!
There are 7 questions waiting for you. Questions are shuffled each attempt.
Take the Quiz