Changing Properties of Matter
Explore how matter changes form through physical mixtures and chemical reactions, and learn to distinguish between reversible and irreversible changes.
1 Physical Changes: Changing Look, Not Identity

Imagine you put on a Halloween costume. 🎭 You look different, right? But are you a different person? No! You are still you. This is exactly how a physical change works.
The 'Big Three' Signs of Physical Change
Melting ice, boiling water, or freezing juice. It looks different, but it is still H2O!
Crumpling paper, crushing a soda can, or molding clay. The shape changes, but the material is the same.
Making a fruit salad or mixing sand with water. You can still separate the parts back out!
Is it Physical? Let's Check!
| Action | Did the substance change identity? | Type of Change |
|---|---|---|
| Chopping Wood 🪓 | No (It's still wood) | Physical |
| Melting Butter 🧈 | No (It's still butter) | Physical |
| Burning Wood 🔥 | Yes (It turned to ash) | NOT Physical |
Key Facts
2 Mixtures: When Substances Just Hang Out

Imagine pouring M&Ms into a bowl of popcorn. Did the M&Ms turn into popcorn? Did the popcorn turn into chocolate? No! They are just hanging out together in the same bowl. 🍿🍫
🧪 What is a Mixture?
A mixture is a physical combination of two or more substances that are blended together without forming new chemical bonds. The most important rule is: Each substance keeps its own identity and properties.
In these mixtures, you can clearly see the different parts. They are not evenly mixed.
- 🥗 Salad: Lettuce, tomatoes, and cucumbers are distinct.
- 🏖️ Sand & Water: The sand sinks to the bottom.
These are mixed so thoroughly that they look like just one substance.
- 🍋 Lemonade: Sugar, water, and lemon juice blend perfectly.
- 💨 Air: A mixture of invisible gases like oxygen and nitrogen.
Because mixtures are just physical combinations, they can usually be separated again using physical methods like filtering, evaporation, or just picking things out with your hands! 🤏
Key Facts
3 Solutions: The Special Kind of Mixture
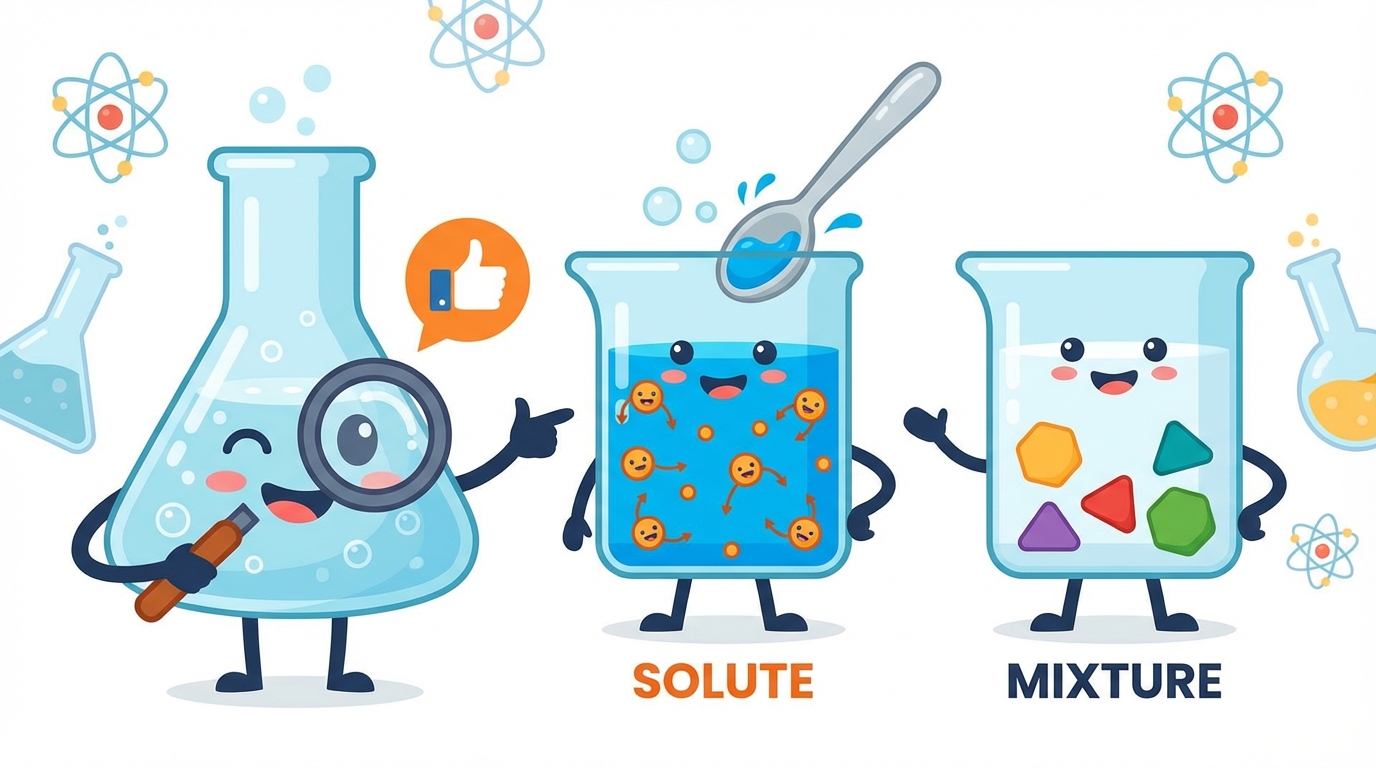
🧪 What is a Solution?
A solution is a special type of mixture where one substance dissolves completely into another. Unlike a salad where you can pick out the tomatoes, in a solution, the parts mix so well that they look like a single substance!
Every solution is made of two players working together:
- 1. The Solute: The substance that gets dissolved (like sugar or salt). Think of it as the guest! 🧂
- 2. The Solvent: The substance that does the dissolving (usually a liquid like water). Think of it as the host! 💧
Solute + Solvent = Solution
Example:
🔍 Mixture vs. Solution
| Feature | Standard Mixture (Heterogeneous) | Solution (Homogeneous) |
|---|---|---|
| Appearance | Looks chunky or uneven 🥗 | Looks the same throughout 🥃 |
| Separation | Easy to separate by hand 🖐️ | Hard to separate (needs evaporation) 🔥 |
| Example | Trail Mix, Sand & Water | Lemonade, Salt Water |
Key Facts
4 Separating Mixtures: Getting Things Back

🕵️ Mixture Detectives!
Have you ever wanted to un-mix something? Because mixtures are physical combinations (not chemical), we can usually separate them back into their original parts using their physical properties!
Used to separate solids of different sizes.
Example: Using a sieve to separate clear sand from seashells at the beach, or sifting flour for baking.
Used to separate a solid from a liquid.
Example: A coffee filter lets the liquid coffee pass through but traps the solid grounds. Or draining pasta with a colander!
Used to separate magnetic metals from non-magnetic materials.
Example: Pulling iron filings out of sand, or recycling centers separating steel cans from plastic.
Used to separate a solid dissolved in a liquid.
Example: If you leave salt water out in the sun, the water turns to gas (vapor), leaving the salt crystals behind.
🧪 The Separation Toolkit
| The Mixture | The Property Used | The Tool |
|---|---|---|
| Sand & Water | Size | Filter Paper |
| Salt & Water | State of Matter | Heat (Evaporation) |
| Iron & Plastic | Magnetism | Magnet |
| Rocks & Sand | Size | Sieve/Screen |
Key Facts
5 Chemical Changes: Creating Something New
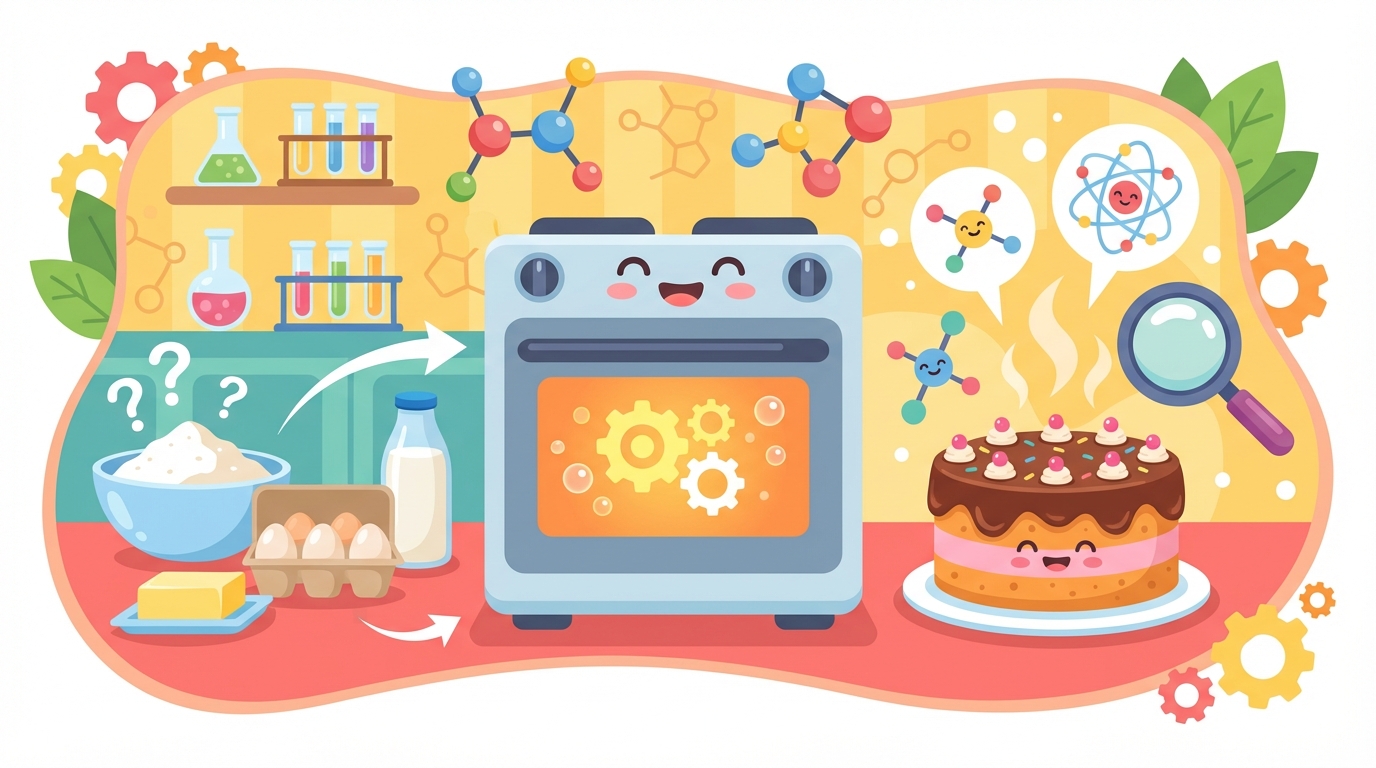
Have you ever wondered why a baked cake looks and tastes so different from the raw eggs and flour used to make it? That is the magic of a Chemical Change! ✨
🕵️ How to Spot a Chemical Change
Since we can't see atoms rearranging, we look for clues! If you see these signs, a chemical reaction is likely happening:
🔥 Heat or Light
Energy is released or absorbed. Think of a campfire burning wood into ash.
🫧 Gas Bubbles
Bubbles appearing without boiling. Like mixing vinegar and baking soda!
🎨 Color Change
Unexpected color shifts. Like a shiny bike rusting and turning orange.
👃 Smell Change
New odors are produced. Like milk going sour or bread baking.
Key Facts
6 Detective Work: Signs of a Chemical Reaction

Put on your detective hat! 🕵️♀️ How do we know if a chemical change has actually happened? We can't always see atoms rearranging, but we can look for clues or evidence.
The 5 Main Clues 🔍
1. Gas Production 🫧
Do you see bubbles or fizzing? That is called effervescence. It means a gas is being made!
Example: Baking soda + Vinegar = Bubbles (Carbon Dioxide).2. Energy Change 🔥
Did it get hot, cold, or make light? Temperature changes or glowing light are big clues.
Example: A glow stick lighting up or a campfire giving off heat.3. Color Change 🎨
If two things mix and turn a totally unexpected color, a reaction happened.
Example: A shiny metal bike turning reddish-brown (Rust).4. Odor Change 👃
A new smell indicates a new chemical.
Example: Milk going sour or an egg rotting.🧪 What is a 'Precipitate'?
This is a tricky one! A precipitate is when two liquids mix together and suddenly create a solid that sinks to the bottom. It looks like magic, but it's chemistry!
Key Facts
7 Everyday Chemistry: Rusting and Tarnishing
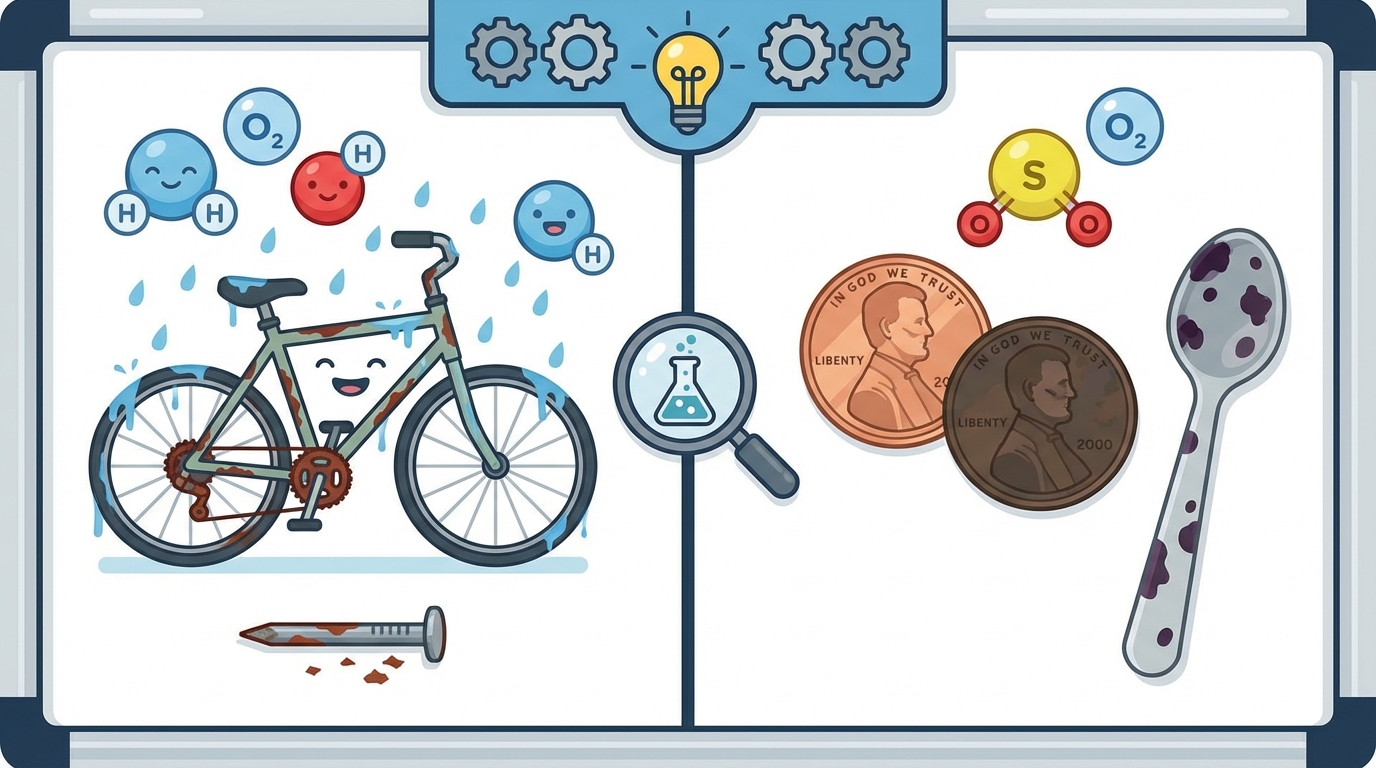
Have you ever left a bicycle out in the rain? 🚲🌧️ Or noticed that an old penny looks dark brown instead of shiny copper? These are examples of chemical changes happening right before your eyes!
🧱 Rust (Iron Oxide)
Rust is a reddish-brown substance that forms on iron and steel. It is a chemical reaction that needs three things:
- Iron (the metal)
- Oxygen (from the air)
- Water (moisture or rain)
Iron + Oxygen + Water ➡️ Iron Oxide
Rust is destructive. It is crumbly and weak, causing the metal to break apart over time.
🥄 Tarnish (Corrosion)
Tarnish is a thin layer of corrosion that forms over metals like silver, copper, and brass. It usually looks dull, gray, or black.
When Silver reacts with sulfur in the air (or in foods like eggs!), it turns black. Unlike rust, tarnish is often just on the surface and protects the metal underneath from further damage.
🔍 What's the Difference?
| Feature | Rusting 🧱 | Tarnishing 🥄 |
|---|---|---|
| Metals affected | Iron, Steel | Silver, Copper, Brass |
| Appearance | Reddish-brown flakes | Dull gray or black film |
| Result | Destroys the metal (weak) | Makes it dirty (can be cleaned) |
Key Facts
8 Heat and Light: Combustion and Cooking
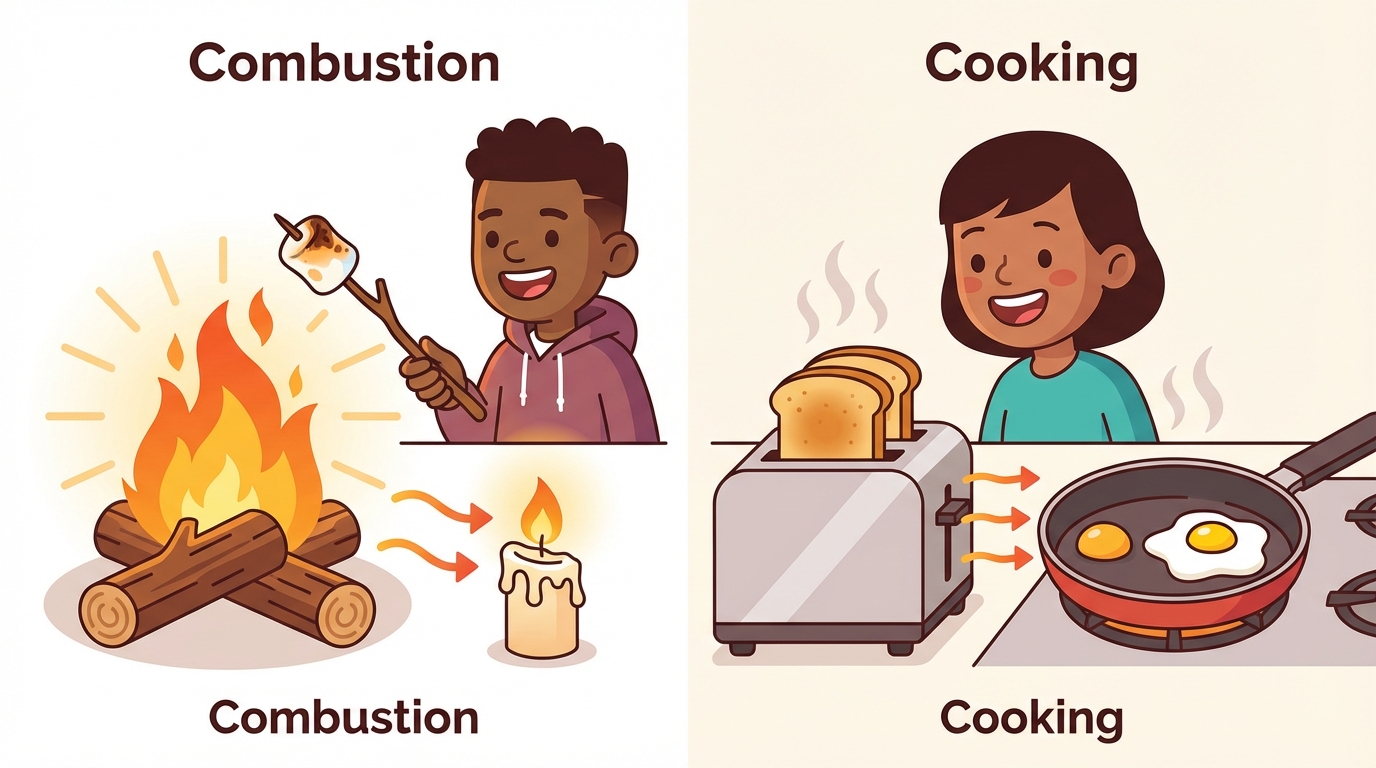
Have you ever stared at a campfire or watched bread turn into toast? You were witnessing chemical changes in action! Heat and light are powerful forms of energy that can completely transform matter.
🔥 Combustion: The Science of Burning
Combustion is a chemical reaction that produces heat and light. For a fire to start, it needs the Fire Triangle:
- Fuel (like wood or gas)
- Oxygen (from the air)
- Heat (to start the reaction)
🍳 Cooking: Edible Chemistry
Your kitchen is actually a science lab! Applying heat to food causes chemical changes that alter texture, color, and taste.
- 🥚 Eggs: Clear liquid turns into a white solid.
- 🍞 Toast: White bread turns brown and crunchy (carbonization).
- 🥩 Meat: Changes from red to brown and develops flavor.
Just like burning, you can't 'uncook' an egg. The heat has permanently changed the proteins!
💡 Light Energy
Combustion doesn't just create heat; it creates light! Before electricity, people used the chemical energy in candles and oil lamps to see in the dark. The energy stored in the wax is released as bright light.
Key Facts
9 Reversible vs. Irreversible Changes

Have you ever wished you could hit an 'Undo' button in real life? In science, some changes allow us to do exactly that, while others are permanent!
❄️ Reversible Changes
A reversible change is a change that can be undone or reversed. The substance might look different, but it is still the same material.
- Melting Ice: If ice melts into water, you can freeze it back into ice.
- Dissolving Salt: If you mix salt in water, you can boil the water away to get the salt back.
- Folding Paper: You can unfold it (though it might be crinkly!).
🔥 Irreversible Changes
An irreversible change is permanent. Once the change happens, you cannot get the original materials back. These often create new substances.
- Baking a Cake: You cannot separate the eggs, flour, and sugar once baked.
- Burning Wood: Wood turns into ash and smoke. You can't turn ash back into a log.
- Rusting: When iron rusts, the metal has chemically changed.
🧪 The 'Undo' Test
To decide if a change is reversible, ask yourself: 'Can I get the original material back easily?'
| Action | Result | Type of Change |
|---|---|---|
| 🍫 Melting Chocolate | Liquid Chocolate | Reversible |
| 🍳 Frying an Egg | Cooked Egg | Irreversible |
| 🎈 Popping a Balloon | Broken Rubber | Irreversible (mostly!) |
| 🧊 Freezing Juice | Popsicle | Reversible |
Key Facts
10 Key Vocabulary
Master these important terms for your exam:
| Term | Definition |
|---|---|
|
Matter
Materia |
Anything that has mass and takes up space.
Todo lo que tiene masa y ocupa espacio. |
|
Physical Property
Propiedad física |
A characteristic of matter that can be observed or measured without changing its identity.
Una característica de la materia que se puede observar o medir sin cambiar su identidad. |
|
Chemical Property
Propiedad química |
A characteristic that describes a substance's ability to change into a new substance.
Una característica que describe la capacidad de una sustancia para transformarse en una nueva sustancia. |
|
Physical Change
Cambio físico |
A change in size, shape, or state of matter that does not create a new substance.
Un cambio en el tamaño, la forma o el estado de la materia que no crea una nueva sustancia. |
|
Chemical Change
Cambio químico |
A process where one or more substances change into entirely new substances with different properties.
Un proceso donde una o más sustancias se transforman en sustancias totalmente nuevas con propiedades diferentes. |
|
Phase Change
Cambio de fase |
A reversible physical change that occurs when a substance changes from one state of matter to another.
Un cambio físico reversible que ocurre cuando una sustancia pasa de un estado de la materia a otro. |
|
Melting Point
Punto de fusión |
The temperature at which a solid changes into a liquid.
La temperatura a la cual un sólido se convierte en líquido. |
|
Boiling Point
Punto de ebullición |
The temperature at which a liquid changes into a gas.
La temperatura a la cual un líquido se convierte en gas. |
|
Solubility
Solubilidad |
The ability of a substance to dissolve in another substance.
La capacidad de una sustancia para disolverse en otra sustancia. |
|
Density
Densidad |
The amount of mass in a given volume; how tightly packed the particles are.
La cantidad de masa en un volumen dado; qué tan compactas están las partículas. |
|
Reactant
Reactivo |
A substance that is present at the start of a chemical reaction.
Una sustancia que está presente al inicio de una reacción química. |
|
Product
Producto |
A substance produced during a chemical reaction.
Una sustancia producida durante una reacción química. |
|
Precipitate
Precipitado |
A solid that forms from a liquid during a chemical reaction.
Un sólido que se forma a partir de un líquido durante una reacción química. |
|
Law of Conservation of Mass
Ley de conservación de la masa |
The rule that matter is not created or destroyed during a chemical or physical change.
La regla de que la materia no se crea ni se destruye durante un cambio químico o físico. |
|
Thermal Energy
Energía térmica |
The energy of moving particles in matter; adding or removing it causes changes in state.
La energía de las partículas en movimiento en la materia; agregarla o quitarla causa cambios de estado. |
|
Flammability
Inflamabilidad |
The ability of a substance to burn.
La capacidad de una sustancia para arder o quemarse. |
Time to Practice!
There are 7 questions waiting for you. Questions are shuffled each attempt.
Take the Quiz